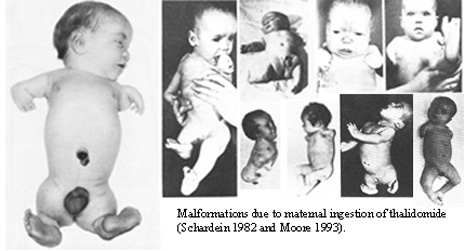
In 1953, the Swiss pharmaceutical company, CIBA, synthesized a new medication which was later obtained by Chemie Grunenthal, a German pharmaceutical company. Grunenthal labeled this medication Thalidomide, and obtained a patent for it in 1954. Just two years later, in 1956, Thalidomide was officially distributed to the public.
Thalidomide was originally developed as a tranquilizer. The seemingly harmless medication quickly grew in popularity, and was used in treating a wide range of conditions including colds, flus, and nausea. It was labeled a "cure all," but was widely used to alleviate morning sickness in pregnant women. Thalidomide ended up being distributed in a total of 46 countries.