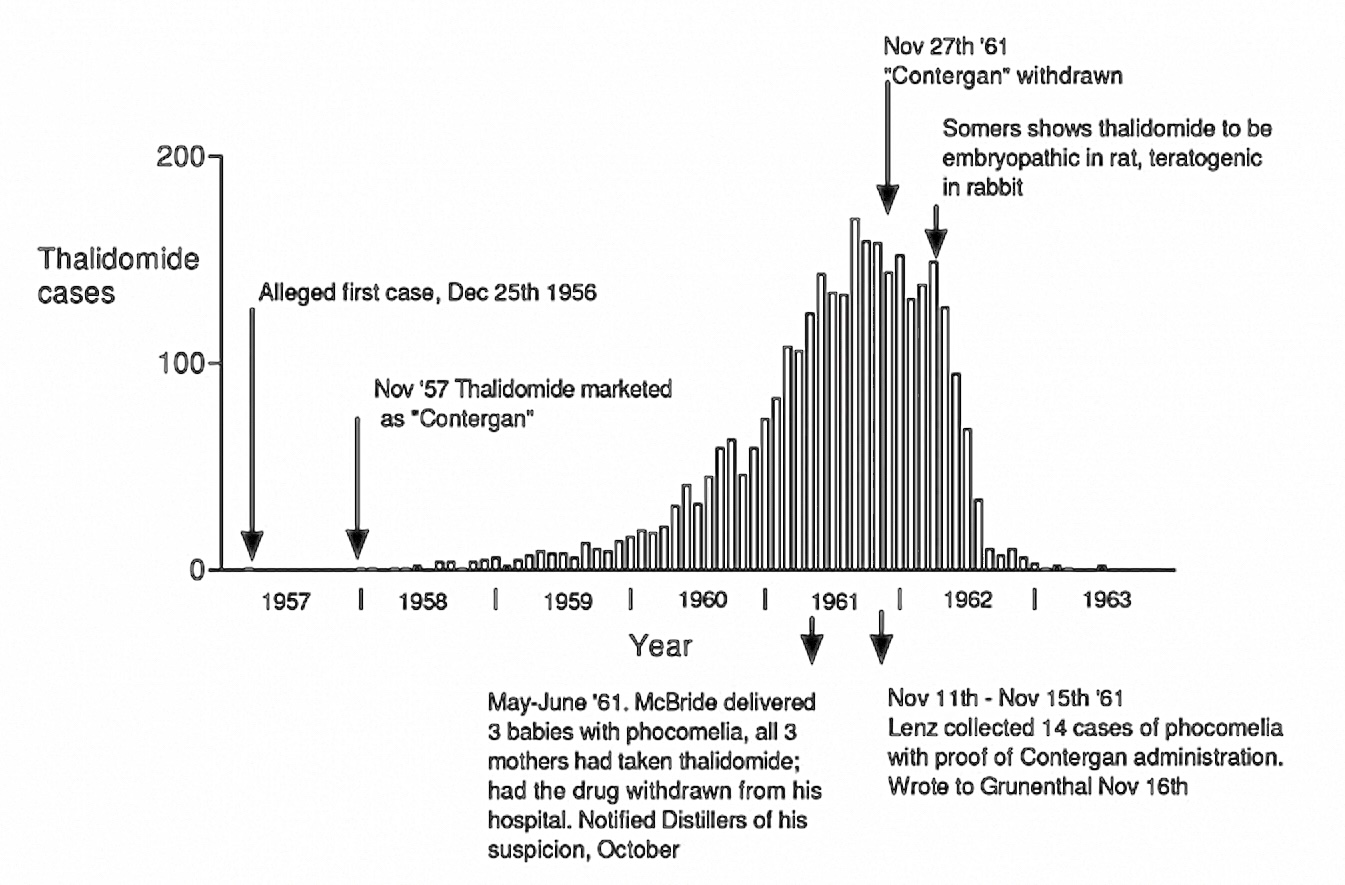
Unethical Marketing
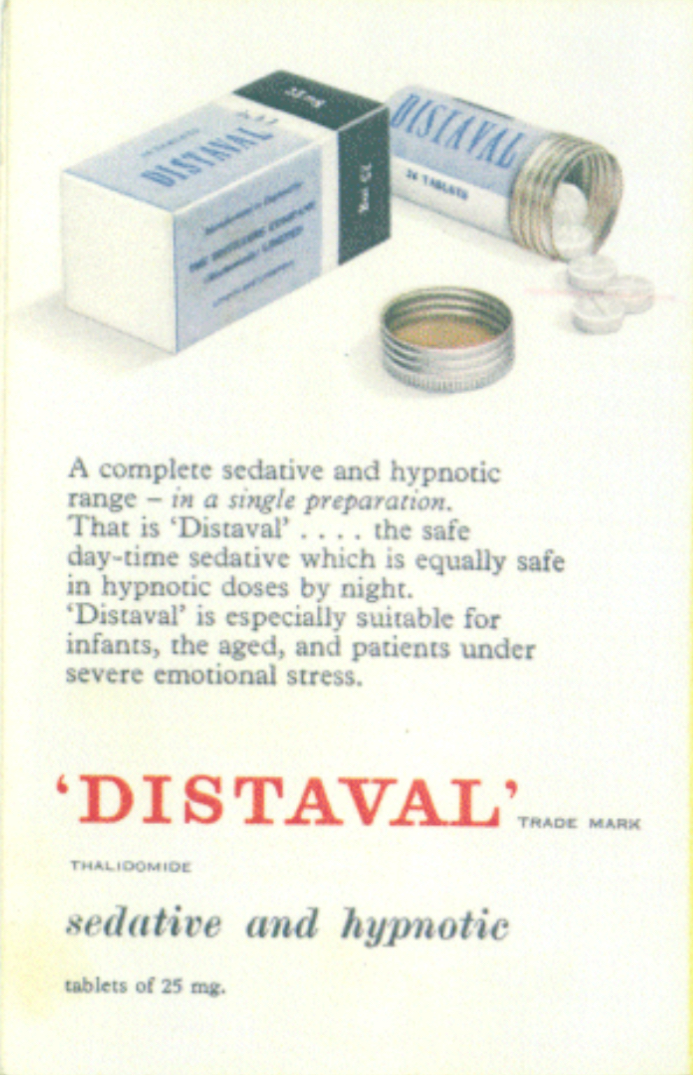
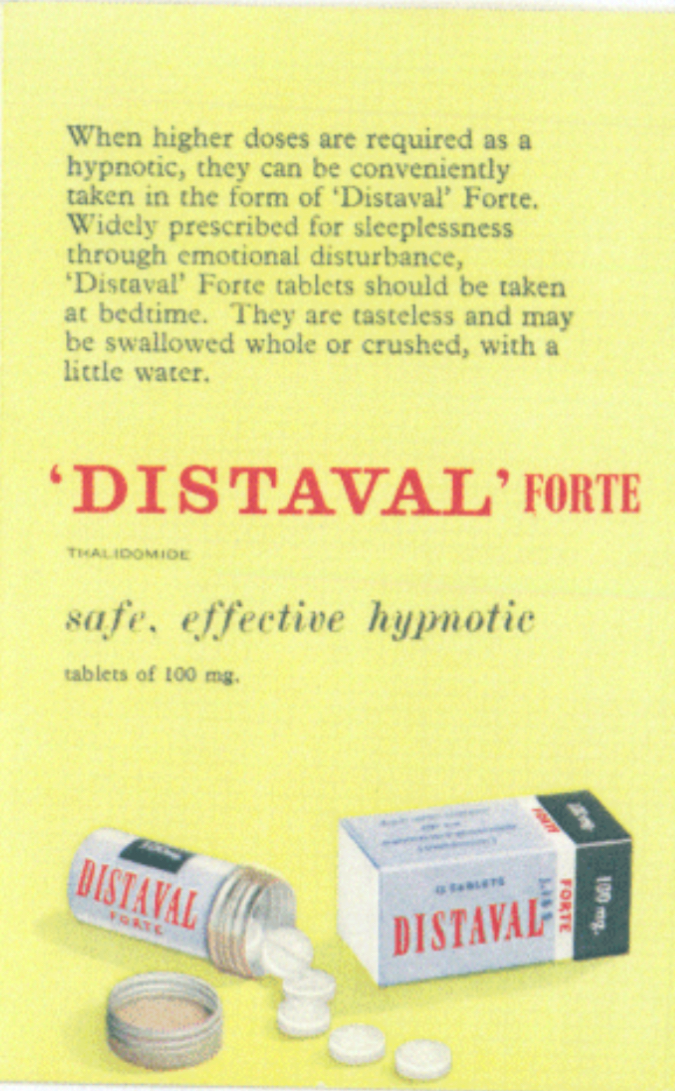
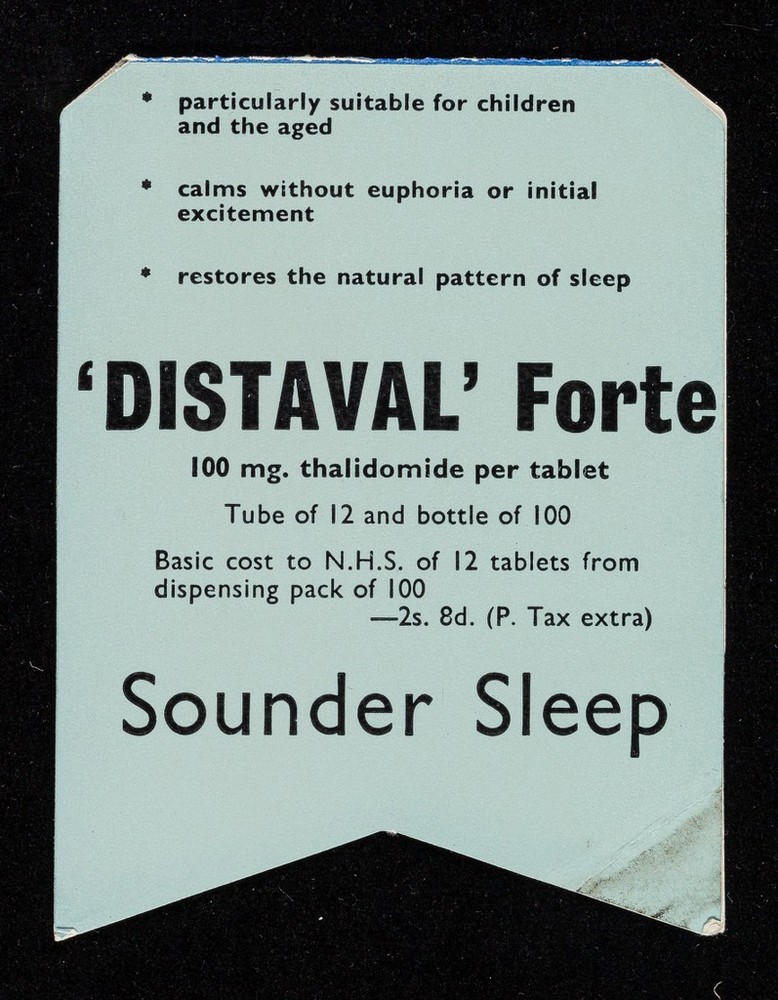
Thalidomdide distributors began unethically marketing and promoting the medication. It was consistantly marketed as being 'safe', and having little to no side effects. There was little testing done to verify these claims. At the time, there were no requirements for companies to complete and fulfill testing protocols. The regulation that was enforced was simple and flawed.
“Distaval can be given with complete safety to pregnant women and nursing mothers without adverse effect on mother or child.” - The Distillers Company (Biochemicals) Ltd, 2020